
Anna Krichevsky, PhD
Professor of Neurology/Neurobiology
Brigham and Women’s Hospital
Harvard Medical School
Research
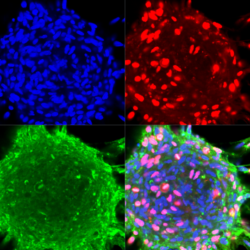
Starting in 2005, our work established miRNA association with all aspects of cancer initiation and progression, including cell proliferation, renewal, migration, invasion, and angiogenesis. Our research focuses on malignant brain tumors (glioma)- a devastating disease and major unmet need in oncology. We have identified the key miRNAs regulating various signaling pathways underlying glioma progression, including miR-21, miR-296, miR-148a, and miR-10b. One striking example is miR-10b, a unique oncogenic miRNA that is highly expressed in all GBM subtypes, while absent in normal neuroglial cells of the brain. miR-10b inhibition strongly impairs the proliferation and survival of cultured glioma cells, including glioma-initiating stem-like cells (GSC). It also attenuates the growth and progression of established intracranial GBM in animal models. Moreover, GBM is strictly “addicted” to miR-10b, and miR-10b gene ablation by CRISPR/Cas9 editing system is lethal for glioma cell cultures and established intracranial tumors. These data indicate that miR-10b is a strong candidate for the development of targeted therapies against various GBM subtypes. Despite its critical role in gliomagenesis, neither the mechanisms of miR-10b induction nor its signaling is sufficiently investigated, representing an exciting avenue for our ongoing work. The work in the lab focuses on the 1) epigenetic mechanisms underlying miR-10b repression in the brain and activation in brain tumors; 2) molecular mechanisms of glioma addiction to miR-10b, and 3) development of ASO and gene editing based strategies and delivery technologies for the therapeutic miRNA/ncRNA targeting in brain tumors.
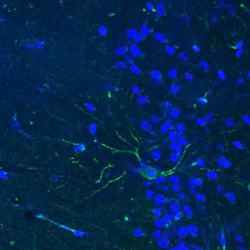
In the nervous system, miRNAs and other ncRNAs play essential roles in development, metabolism, stress response, neuronal plasticity, and survival. Dysregulated gene expression and RNA metabolism have been observed in various neurologic diseases. Our work implicated miRNAs in Alzheimer’s disease (AD) and other neurodegenerative disorders. For example, a specific miRNA, miR-132, emerged as a critical regulator of synaptic and neuronal health that is commonly dysregulated in AD and other neurodegenerative diseases ranging from Frontotemporal Dementia (FTD) and Progressive Supranuclear Palsy (PSP) to Huntington and Parkinson diseases (HD and PD, respectively). miR-132 regulates the metabolism of tau, one of the key factors contributing to neurodegeneration and dementia, and supplementation of this miRNA rescues neuropathology in cell and animal models. The ongoing work in the laboratory employs human iPSC-derived neural cells and animal models of neurodegeneration. It focuses on the 1) revealing the targetable hubs associated with RNA pathologies, and 2) development of diverse therapeutic strategies for targeting regulatory RNA species in the brain and CNS.
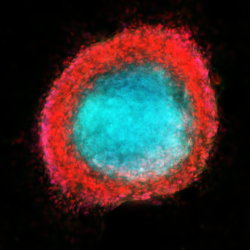
Extracellular vesicles and extracellular RNA emerged in recent years as a new means of intercellular communications and robust biomarkers for diverse pathologies. We demonstrated that CSF-based signature miRNAs identify glioblastoma and metastatic brain cancers and reflect disease activity, providing new avenues for the development of RNA biomarkers for neuro-oncologic and neurologic disorders. The lab’s ongoing work is also focusing on the exRNA as paracrine signals mediating a cross-talk between the brain tumors and diverse cells of the tumor microenvironment, and the impact of such communication on the growth and recurrence of brain tumors.